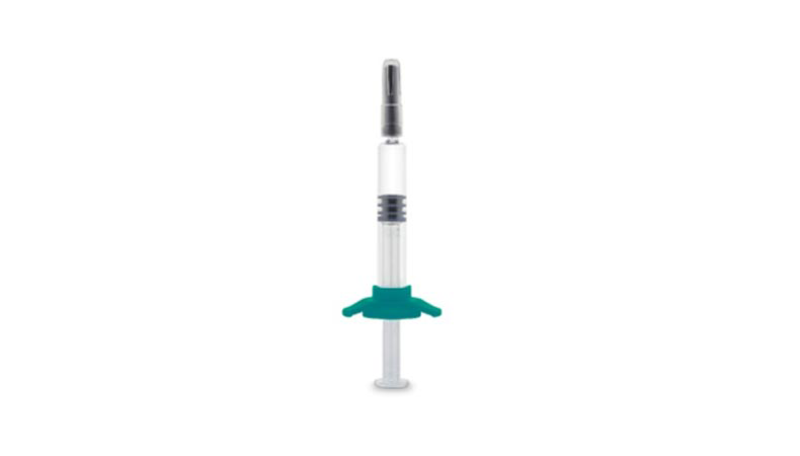
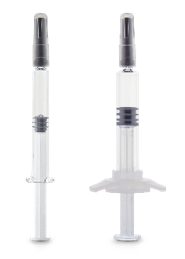
Production for the new 2.25 ml COP (cyclic olefin polymer) SIN (staked in needle) will start this month
Düsseldorf/Paris, January 23, 2020. Gerresheimer is expanding its range of pre-fillable polymer syringes to include a new product: the Gx RTF ClearJect polymer needle syringe, 2.25 ml. Like the 1.0 ml syringe, this syringe will be produced in Pfreimd, Germany. The new product will be showcased at Pharmapack in Paris Booth B60/B64.
The material used for the syringe is a high performance polymer called COP (cyclic olefin polymer). It is suitable for use as primary packaging for sophisticated medications, especially for sensitive, biologicals, biosimilars, and biobetters. The product was developed in close cooperation between two Gerresheimer locations in order to create synergy between the syringe experts in Bünde and the plastic experts in Wackersdorf, Germany.
The Gx RTF ClearJect COP SIN of Gerresheimer Bünde GmbH is now available in the sizes 1.0 ml long and 2.25 ml. The design is inspired by ISO 11040-6 and registered. The syringe is equipped with a 27-gauge, 1/2-inch (12.7 mm), thin-walled stainless-steel needle with three bevels.