High-performance automation solutions for diagnostic and medical devices
Design, development and manufacturing of product-specific automation solutions
Especially for large series production in the diagnostic product and medical product industries, automation coordinated precisely with the product, project and processes has a decisive influence on the quality and economic efficiency of production. The technicians, mechanics, electricians, designers and programmers of the Automation Engineering department are responsible for these tasks at our Technical Competence Center (TCC) in Wackersdorf (Germany). The 80-member team specifies, procures, qualifies, designs and builds systems for special-purpose machinery, automatic testing systems and loading and unloading handling, which are used at all of our production facilities in Europe, the USA, and Asia.
Your PLUS with Gerresheimer
Automation - integral element of product development
Automation is an integral element of our product and process development. Our know-how flows directly into product development during the concept and design phase. Automation solutions are not first formulated for series production, but are instead already developed in the prototype and preproduction phase to save time. The knowledge gained here can also be passed on to other automatic systems manufacturers when an external solution is planned for series production.
Automation solutions worldwide standardized at an uniform level of quality

All of the automation solutions we develop and use are standardized worldwide at a high level of quality. For example, we have optimized all of our handling systems to be GMP-compatible in order to simplify operation and maintenance in the production facility.
One contact for all systems of a project/product

We specify, develop and build the best solution for your product. In the process we utilize our experienced suppliers on the market and build systems ourselves in our internal special engineering department. Our internal qualification department is responsible for the qualification of all systems in the project. For you this means, whether purchased or built ourselves: you have only one contact for all systems of the project.
Most modern means of a production for the diagnostics and medical device industries
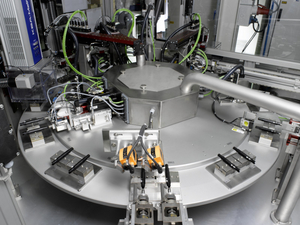
We specify, procure or develop customer and part-specific automatic assembly systems, automatic testing systems (pressure, flow rate, visual features, force deflection systems), rotary table systems, linear systems, robots for the insertion and removal of parts, packaging systems, preproduction equipment and systems for pharmaceutical assembly as well as glass forming lines, cannula assembly and RTF lines. All of the production systems we manufacture fulfill the requirements of GAMP (Good Automated Manufacturing Practice) and FDA 21 CFR Part 11, and are designed for production in clean rooms in accordance with ISO 14644-1 class 7 / 8 or GMP Grade C / D.
