Phase II: Product and process development of drug delivery devices
Your PLUS with Gerresheimer
Definition of the detailed product requirements

We define all relevant European and international, normative, legal, technical, and regulatory requirements of the new product in the Product Requirements Document. The objective of Phase II is to develop the design with regard to the defined requirements extending to the “design freeze” in the context of product development.
Product development
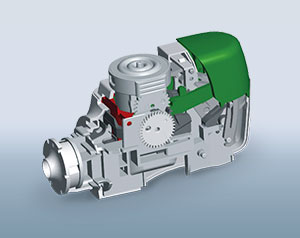
We develop drug delivery devices, combination products and pharmaceutical packaging pursuant to regulations (e.g. MDR 2017/745, ISO 13485, ISO 15378, FDA 21 CFR 820). Our product development process makes it possible for us to react flexibly to the product requirements and to thereby observe the necessary regulatory and normative requirements. Your advantage here is that we have access to many years of experience and closely networked internal capacities. We develop a product for subsequent robust production, whether for a limited number of products for a clinical study, which need to be available quickly, or for high-volume mass production.
Process development
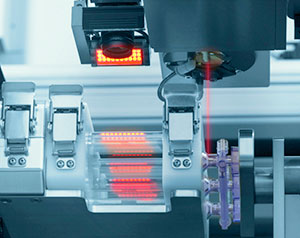
During product development, the necessary assembly and finishing processes are defined as early as possible. Each process is examined in detail in the process and redeveloped as required. We consult closely with you and with our internal experts and external suppliers to find the best solution. Based on this, optimal processes are created, which fulfill the requirements for both robust production and for quality. We also analyze the processes for potential patenting.
Simulations and tests
With product and function-specific simulations, we assure producibility in plastics, resistance to mechanical stresses, subsequent highly automated assembly, and testing capability. By way of upstream injection molding simulations like Moldflow, we eliminate weak points and material accumulations and determine optimal injection points, as well as process stability injection parameters for the plastic parts. With the help of the Finite Element Method, we determine and optimize the structural behavior of the components. Statistical tolerance analyses show the influence of fluctuations of production parameters on the function of products. Multi-body and flow simulations round off our service portfolio. If test results and simulations lead to adjustments to the design or material, these improvements are implemented hand-in-hand with tool and special engineering.
Creation of functional samples, preselection of suppliers, and selection of material
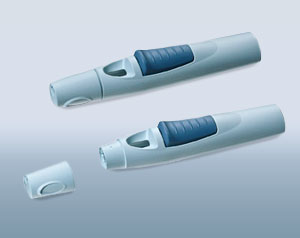
Samples of the subsequent final product can be created at any time in the project. The farther along a project has progressed, the more precisely the actual properties of the components can be reproduced and the functions tested. In Phase II, parts made of the original material, which were manufactured with the injection molding process, are already tested. The materials are selected on the basis of the requirements for the product and the component in consultation with the customer and our technology experts, as well as with preselected suppliers and manufacturers of the materials.
Packaging design
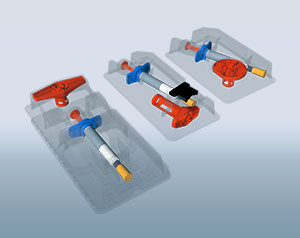
We create innovative packaging concepts that not only optimally protect your drug delivery devices, but also at the same time address the needs of users.
