
Medical Devices
For customized medical products and medical devices we are a reliable cooperation partner for the development from the idea to series production-readiness that combines innovative strength and industrialization competence. On behalf of our customers, we develop and produce medical products and medical devices like, for example, lancing devices, infusion sets, disposable systems for a hemostatic scalp clip system and for artificial respiration. We also manufacture disposables for trocars, components for blood pumps, as well as operation grips of plastics. In the process we specialize equally in fully automated large series production and in the manual and semi-automatic small series production of complex, technically sophisticated injection molding products.

Experience the Synergy: Medical Devices and Electronics
The partnership between Gerresheimer and Zollner is based on a clear vision: to offer customers along the entire value creation chain a comprehensive service offering – from concept development through electronic integration to series production. Using the example of a medication pump for Parkinson therapy, we demonstrate in our newsletter the advantages of our integrated value creation chain.
As a full-service provider, Gerresheimer Medical Systems assumes responsibility for all levels of the value creation chain, from the initial product idea for a medical product / medical device through assembly to the finished CE-marked medical product: Concept development, industrial design, product development, manufacturing equipment design, mold making, automation engineering, large and small series production in the clean room according to ISO 14644-1 ISO classes 7, 8, and 9 and GMP classes C and D, product finishing, as well as manual, partially automated and fully automated assembly, picking and packaging. We assume responsibility for the installation of the entire electronics into the end devices and organize the various purchase parts. In this process we make use of strategic partners subject to regular quality audits and that fulfil our strict requirements 100 %.
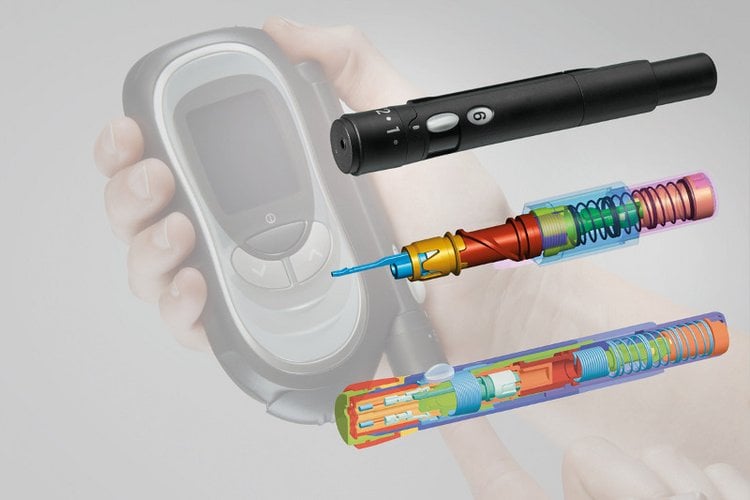 Development of a lancing device
Development of a lancing deviceWe develop your medical device or medical product made of plastics in accordance with user requirement specifications or optimize the component layout of existing customer ideas for injection molding production. The Gerresheimer Medical Systems engineers allow the design for manufacturing to flow into the development of new medical devices right from the beginning. For our customers this means a reduction in the development time, of development costs and of project risk, as the step of plastic-compatible optimization is dispensed with. Our experts for the design and development of medical devices made of plastics attach importance to product-specific requirements like, for example, special gluing techniques for a burr-free, blood-friendly surface, the connection of metal and plastics, as well as the implementation of electronics components.
 Design for Manufacturing flows into the development of medical devices
Design for Manufacturing flows into the development of medical devicesIrrespective of the number of units and the requirements of the medical device, we offer our customers a tailored production solution. In addition to the fully automated large series production of medical devices, we also realize the manual and semi-automatic small series production of complex, technically sophisticated injection molded products.
 Assembly and application of Teflon to the slipway of a medical device
Assembly and application of Teflon to the slipway of a medical deviceGerresheimer Medical Systems occupies a leading position worldwide in the production of medical devices. This is documented by an FDA inspection resulting in no objections whatsoever and classification as an "FDA-inspected device manufacturer". The audit on the occasion of the production of the "CentriMag®" blood pump system" covered the entire value creation chain. In this case Gerresheimer Medical Systems assumed responsibility for the injection molding, assembly, testing, and sterile packaging in the clean room according to ISO 14644-1 of ISO class 8 and hygiene monitoring in accordance with GMP class D.
 CentriMag® blood pump system: Audit of the entire value creation chain
CentriMag® blood pump system: Audit of the entire value creation chainOur Technical Competence Center (TCC) in Wackersdorf (Germany) and Peachtree City (USA) offers their own production systems (injection molding machines, project-specific assembly systems like automated joining systems, gluing apparatus or systems for ultrasound welding) on which the quick and uncomplicated production of development samples, clinical samples or small series for your medical product or medical device is possible at any phase of the project. The TCCs possesses a clean room in accordance with ISO 14644-1 ISO-class 7, 8 and GMP classes C and D, in which the entire value creation chain can be reproduced.
 Small series production in the clean room according to ISO 14644-1 ISO-class 8
Small series production in the clean room according to ISO 14644-1 ISO-class 8We offer production facilities in Pfreimd (Germany), Horšovský Týn (Czech Republic), Skopje (North Macedonia), Peachtree City (USA), Indaiatuba (Brazil) and Dongguan City (China), which serve our international medical devices and medical product customers worldwide. With a production area of 120,000 sqm (1,300,000 sqft), of this around 60,000 sqm (670,000 sqft) of clean room area according to ISO 14644-1 ISO classes 7, 8, 9 and GMP classes C and D, as well as more than 350 injection molding machines, we are one of the leading companies in the industry.
 Clean room production in accordance with ISO 14644-1 ISO class 8 in Pfreimd, Germany
Clean room production in accordance with ISO 14644-1 ISO class 8 in Pfreimd, GermanyTogether with a leading diagnostics company, we in the meantime develop and produce lancing devices and lancets for blood sampling in the fifth device generation. In order to enable low-pain blood taking, we have optimized nearly all components of the lancing devices in the last 15 years. Starting with increasingly fine needles, which hardly injure tissue and ensure quick wound healing through a precise feed of the lancets to the ergonomic optimization of the lancing devices, all innovations are aimed at increased user-friendliness and thus at an improvement in compliance.
 Five generations of lancing devices
Five generations of lancing devices