Gx Elite RTF® syringes
Silicone-oil-free syringe systems made from Glass and COP
Our prefillable syringe portfolio for ophthalmology is designed to address the requirements in this specific therapeutic area to ensure safe storage of a variety of sophisticated drug products, improve handling during application and enhancing patient’s safety.
To further support the high standards of this therapeutic area as well as the requirements of next generation drug technologies we are proud to announce the first complete silicone-oil-free syringe systems in the market. Together with our partners in the pharmaceutical industry we thrive to improve patient treatments and wellbeing.
Gerresheimer currently covers around two-third of the demands for pre-fillable syringes used for VEGF-A inhibitor treatments
Why choosing silicone-oil-free syringe systems?
Although silicone oil particles are harmless to the human body, often they are an unfavored byproduct causing risks when injecting drugs intravitreally using prefilled syringes. Especially for new therapeutic drug technologies in the area of Ophthalmics, RNAi or AAV-based gene therapies, silicone oil can also be an issue for the drug product.This is why we at Gerresheimer thrived for solutions to eliminate silicone oil in all for our components used with our glass and polymer syringes.

Benefits of silicone-oil-free syringe systems
To further improve our portfolio we decided to eliminate free silicone oil from all components of our prefillable syringe systems to minimize the risk of drug interaction during storage as well as potential risks at the patient after injection by ensuring best-in the market functionality.
Suitable configurations meeting specific requirements
Both, glass and the high-end polymer COP (cyclic-olefin polymer) syringes are perfectly suited for syringe-based injections in the area of e. g. ophthalmology. Nevertheless both materials have specific benefits to consider when choosing the right configuration for your application.
Gx ELITE RTF® COP syringes


Gx ELITE RTF® Glass syringes



Gx RTF® ClearJect syringes

Gx RTF® Glass syringes
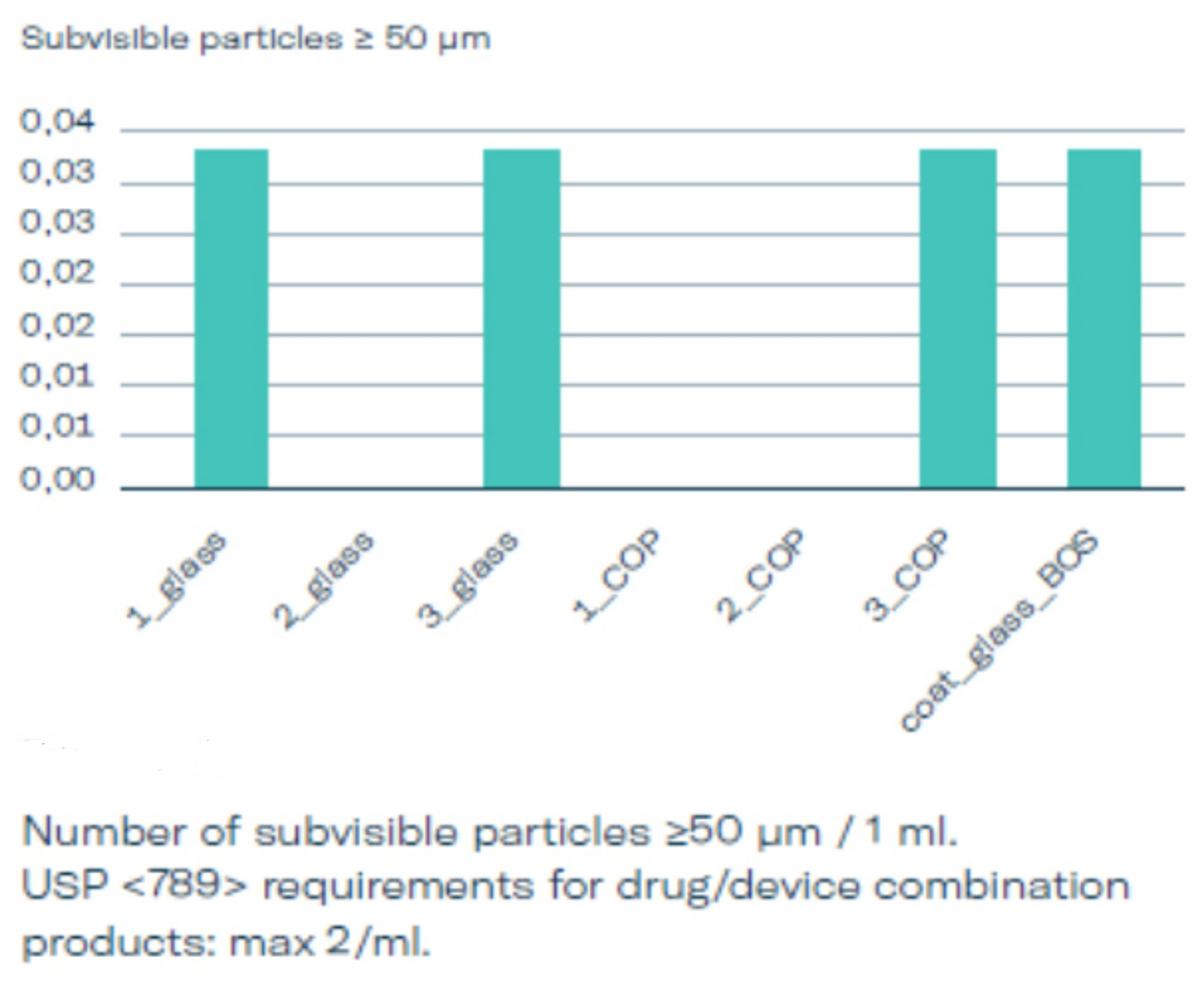
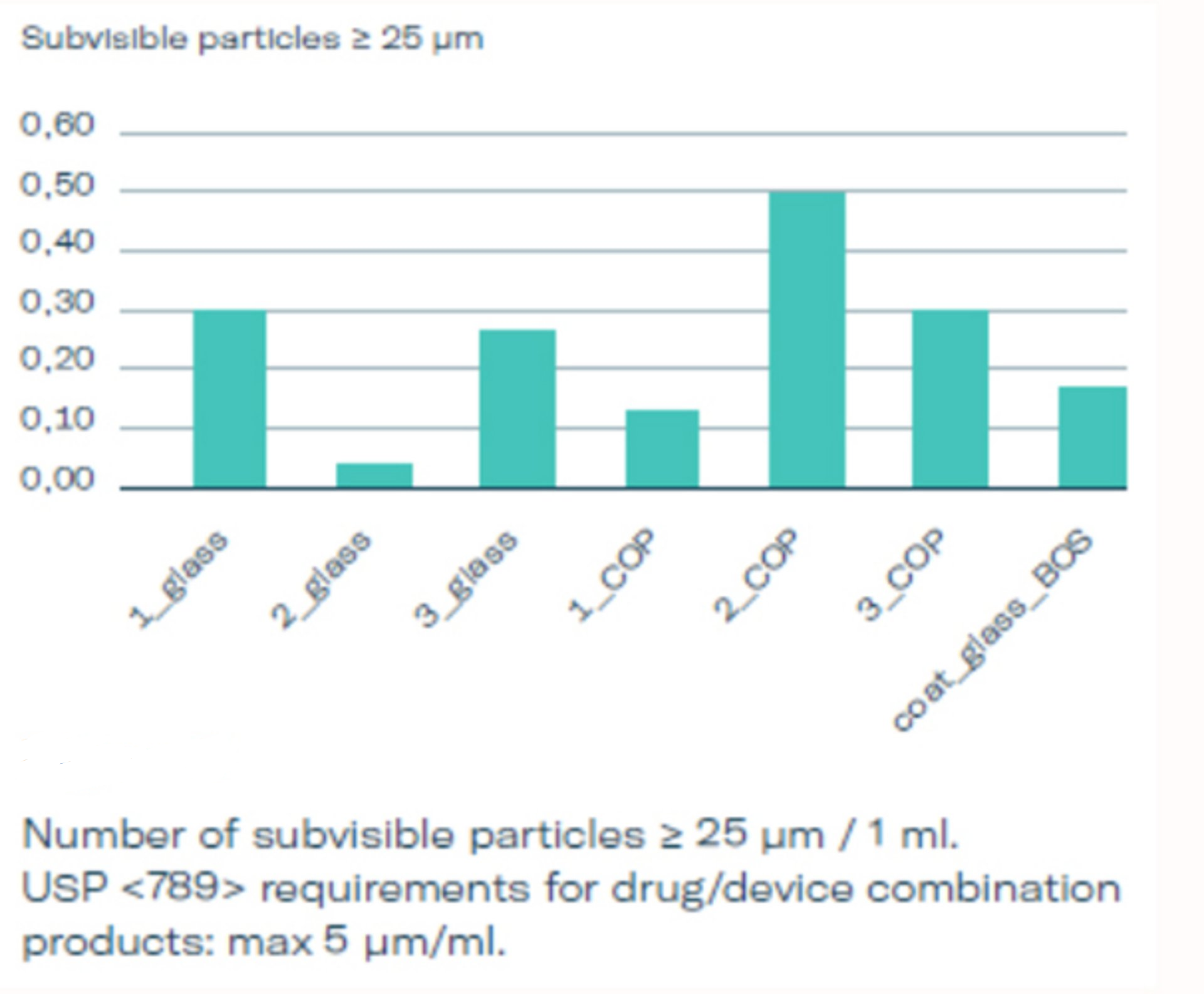
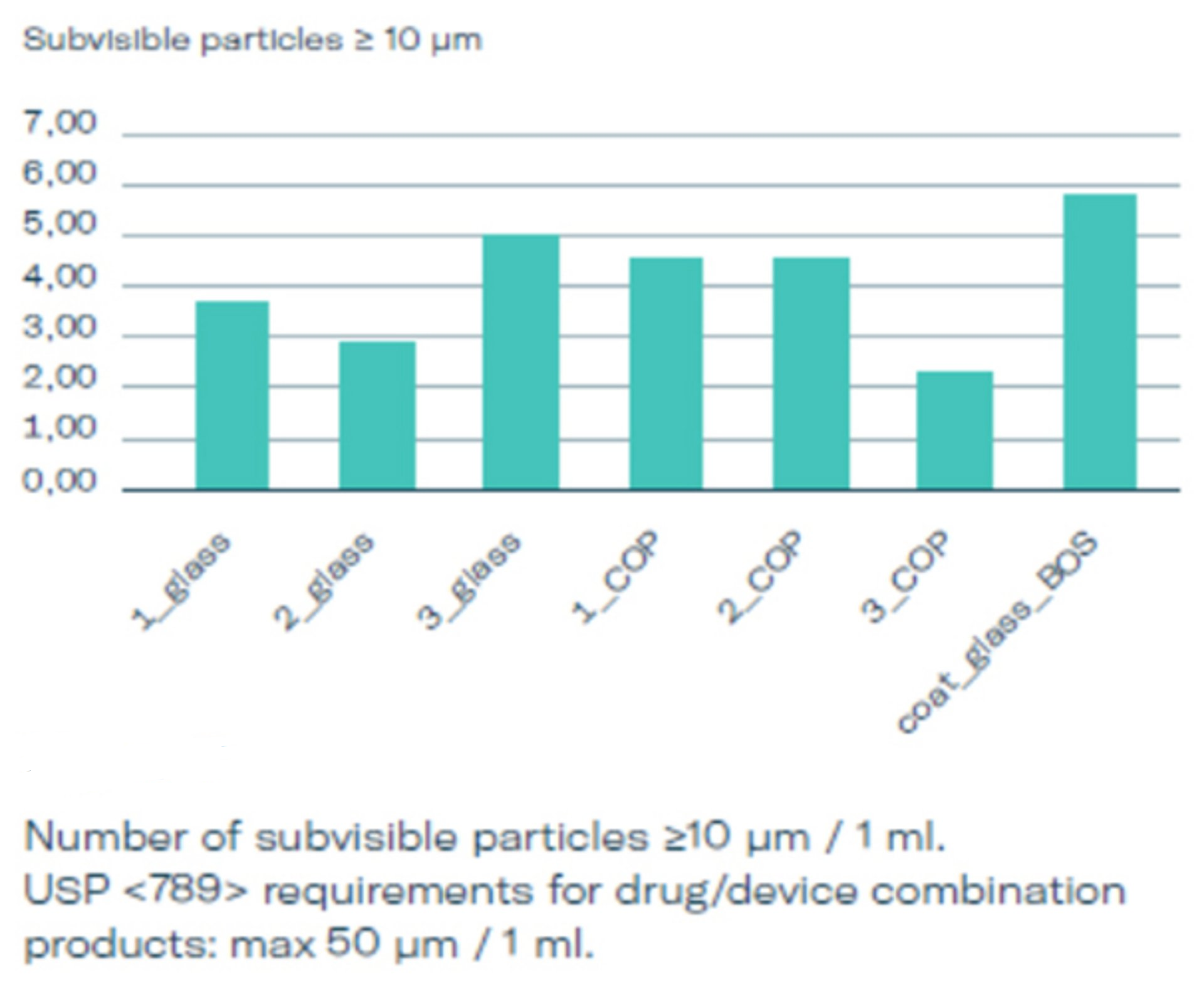
Whitepaper and Testing data: Particles
In a broad set of testing three different stopper options (1, 2, 3) used in glass and COP syringes were evaluated and compared to baked-on siliconized glass syringes. The subvisible particles were measured according to USP <789> which is relevant for the area of ophthalmic injectables after 3 months of real time storage using the light obscuration method, see graphics 2, 3 and 4. The particle count for silicone-oil-free syringes is exceptionally low. BOS syringes have shown particles loads comparable to the silicone-free options, suggesting that both are well suited for ophthalmological applications. Both comply well with USP <789> requirements and can certainly be considered as an alternative for siliconized PFS for ophthalmological injections already existing on the market.

All you need to know about PFS
New possibilities in terminal sterilization for ophthalmics


