Subcutaneous self-administration of biopharmaceuticals
Created for home-use, the Gx Inbeneo® autoinjector enables easy, safe subcutaneous self-administration. With a pre-pressurized, cartridge-based design, the autoinjector can quickly and effectively deliver highly viscous biologic drugs.
Thanks to our platform concept, the Gx Inbeneo® can be easily customized to different drug viscosities and fill volumes up to 3 mL. We have extensive experience and can support your full development including documentation for regulatory submission of your final combination product.

Your new autoinjector for biologics
If you’re developing large molecule drugs for subcutaneous administration, we understand that you’re considering numerous factors when evaluating your new device partner.
When you choose Gx Inbeneo® you can be confident of a safe, reliable, patient-friendly device that can be quickly adapted to your specific drug formulation. What is more Gerresheimer’s expertise in development, manufacturing, and regulatory submission reduces development risk and time-to-market.
Benefits of our Gx Inbeneo® Autoinjector platform
Device Design that stands out
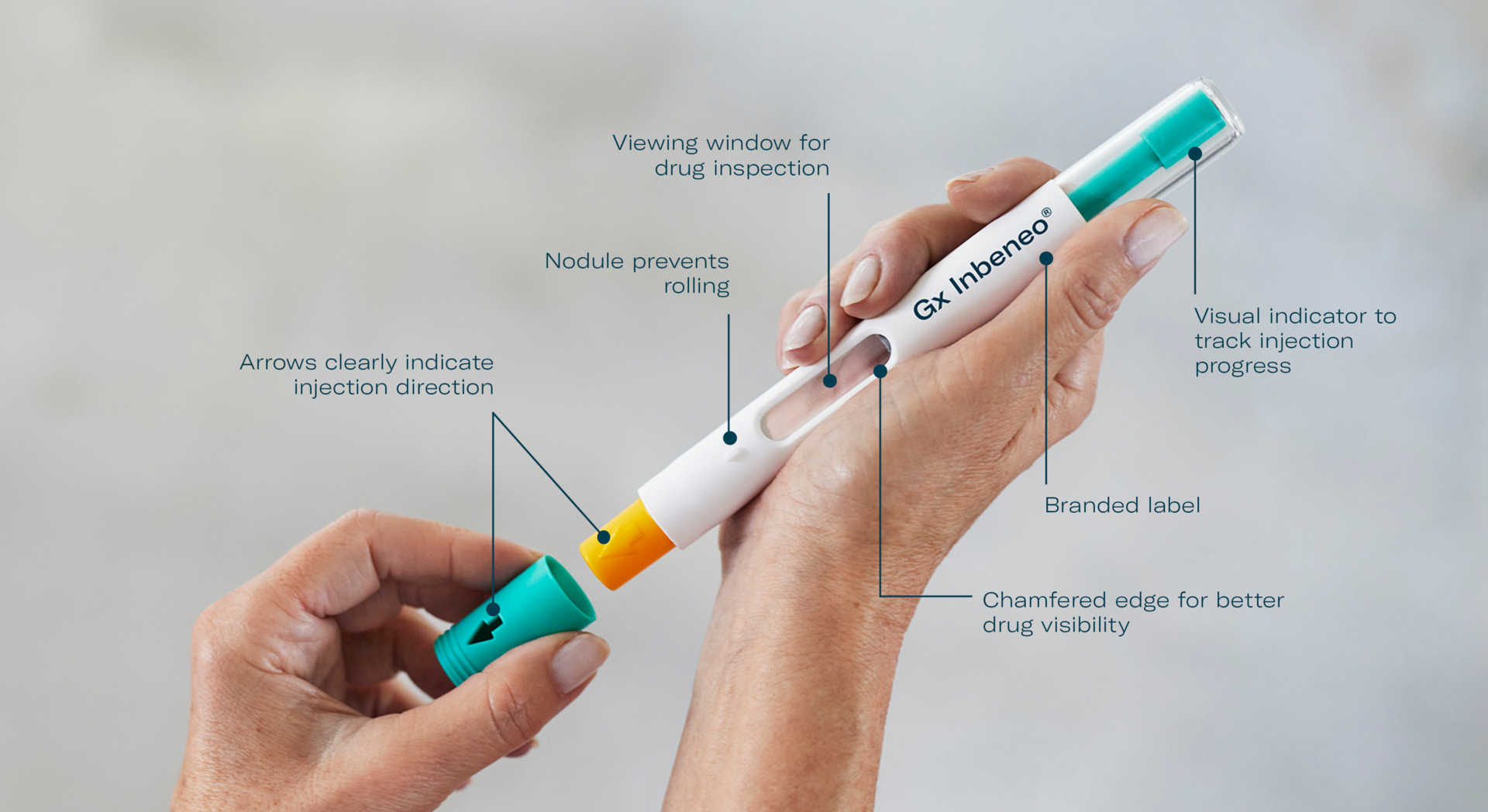

Easy & safe for patients
Quick and simple self-administration enables patients to integrate their therapy into their daily life.
- Reduced risk of needle-stick injury as the needle is already installed and secured within a safety shield before and after usage
- Push-on-skin activation with automatic needle insertion and drug delivery
- Confident tracking of injection progress even if the patient grips the autoinjector with the whole hand, thanks to the transparent top casing
- Reassuring audible end-of-dose click
- Slim and lightweight, yet robust product design



Simple two-step injection
Administration of a therapeutic drug is as simple as 1, 2, done!
The process
The patient simply removes the cap of the Gx Inbeneo® and pushes the end of the autoinjector against their skin. As the safety shield retracts, one end of the needle pierces the cartridge septum and the other end pierces the patient's skin. The fluid pathway is established, and delivery of the drug begins automatically. The patient can observe progress via the central inspection window or transparent top-casing. End-point is further indicated by an audible click. After usage the needle is secured by the safety shield ready for safe disposal.
Meeting the challenges of biologics
Delivering biologic drugs subcutaenously in a home-care setting can be challenging. Sensitive molecules and viscous formulations place demands on the device. The Gx Inbeneo® autoinjector has been purpose-designed to solve these challenges.
Quickly adaptable for reduced time-to-market
The Gx Inbeneo® platform concept from Gerresheimer decreases time-to-market and development risk, thanks to:
- Quick tailoring to specific therapeutic drugs as needle, spring and cartridge can be combined as needed within the same housing
- Full-service from Gerresheimer including manufacture and supply of ISO cartridges of 1.5 or 3 mL
- Customizable cap and plunger colors and options for company and product logo printing


Benefits of a cartridge-based, pre-pressurized design
Pre-filled syringe-based autoinjectors (PFS) employ a compressed spring held in place with a locking mechanism that must be reliably released at the moment of injection. In contrast, the unique pre-pressurized design of the Gx Inbeneo® employs a robust prefilled glass cartridge to retain the spring force, which makes the cartridge the force barrier. As no additional spring locking mechanism is required, any associated risk of failure is eliminated.
This innovative pr-pressurized design enables the use of springs strong enough to inject drug products with viscosities up to 100 cP* at volumes of up to 3 mL, based on calculations.
Early evaluation of device set-up
The drug development process is long and complex. Formulations, concentrations and volumes are adjusted at different stages, which can make it tricky to define device requirements early. Therefore, we have designed the Gx Inbeneo® Platform Simulator together with our partner Midas Pharma. It helps you integrate device definition into the drug development process.
The simulator mimics the Gx Inbeneo® platform with different needle gauges, spring forces, and cartridge sizes available for testing. This enables:
- Quick evaluation of injection time with a specific formulation at any stage of drug development
- Assessment of any impact of injection pressure on the drug
- Precise definition of the prototype design for clinical trial
We can supply the Gx Inbeneo® Platform Simulator for testing at your site.

Primary container | ISO 1.5 or 3 mL cartridge |
Fill volume | 0.5 – 3 mL fill volume |
Viscosity | 0 – 100 cP |
Dimensions | L 195.75 mm x Ø 20 mm (with cap) |
Needle design | Double-ended* |
Patient needle | 25, 27, or 29 G |
Needle insertion | Automatic, push-on-skin activation |
Needle protection | Safety shield covers needle before & after use |
Injection progress indicators | Visual with central window & transparent casing, audible with end-point click |
System type | Pre-pressurized |