Customized point-of-care tests
PoC test cartridge development and manufacturing
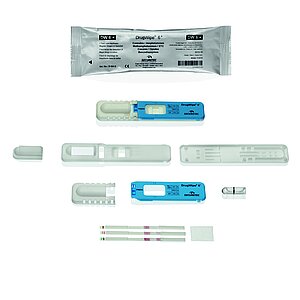
20 years of experience in the production of point-of-care tests make us the ideal partner. Whether COVID19-tests, drug detection, pregnancy tests, detection of emergency parameters or allergy tests, whether use in the doctor's office or in the analysis device in the lab: We develop and produce your complete PCR-test including loading of the point-of-care tests with test strips and filling of the quick test with the necessary dilution solution.

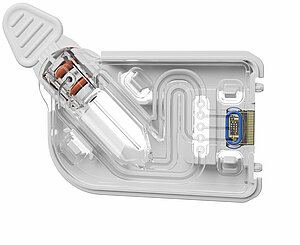
Hermetically sealing micro-channels is considered one of the main challenges in developing diagnostic systems in medical technology. We have extensive process expertise when it comes to sealing microfluidic structures. Learn more by downloading our newsletter how we support our customers in selecting and implementing a suitable joining method during the development and industrialization phase. Discover how we design seals that are mechanically stable, resistant to chemicals, optically clear and cost-effective.
Process expertise plays a crucial role in the sealing of microfluidic structures in medical technology

Your PLUS with Gerresheimer
Full service: from the concept development to the finished PCR-test
Gerresheimer Medical Systems assumes responsibility for all stages of the value creation chain: from the initial concepts for point-of-care test housing made of plastics extending to the packaging of sensitive end products: Concept development, industrial design, product development, manufacturing equipment design, mold making, automation engineering, large and small series production under FDA/GMP conditions, high quality printing of the quick test housing secured through camera testing, as well as manual, semi-automated and fully automated assembly of the housing components of the PCR-test and of the test strips, filling of the required reagents, packaging, and international logistics. We offer our customers highly precise production, including the complicated cleaning steps in the clean room in accordance with ISO 14644-1 ISO classes 8 and 9.
Design for Manufacturing: reduction of development time
We develop your point-of-care test in accordance with user requirement specifications or optimize the component layout of existing customer ideas for injection molding production. The Gerresheimer Medical Systems engineers allow the Design for Manufacturing to flow into the development of new point-of-care tests made of plastics right from the beginning. For our customers this means a reduction of development time for PCR-tests, of development costs and of project risk, as the step of plastic-compatible optimization is dispensed with.
Optimal housing design for your point-of-care test
Upon commencement of development, we select the plastic material ideal for the test in order to ensure the flexibility of the PCR-test. Our experts for quick tests then develop a customer-friendly housing design with simple and comprehensible handling. Optimal connection technology (snap lock, gluing or welding) and optimal functional support of the membrane (pressure points) complete the development of a user-friendly and safe point-of-care tests.
Microstructured mold parts for PCR tests
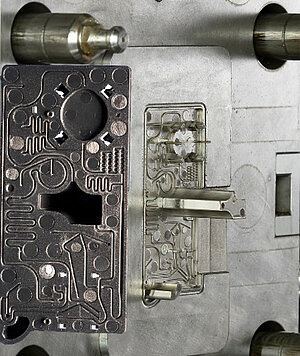
We offer our diagnostics customers experience not only in micro components, but also in microstructured plastic mold parts for microfluidic test systems. The prerequisite for high quality microstructured mold parts for PCR-tests is extremely precise molds. A component tolerance of less than 30 µm, for example, presumes mold tolerances of less than 10 µm. In our mold making section we therefore use machinery especially conceived of for this task. The removal of plastic parts following the injection mold requires a great deal of sensitivity in order to avoid damaging the fine channels.
Small series production of quick tests
Our Technical Competence Center (TCC) in Wackersdorf (Germany) and Peachtree City (USA) offers their own production systems (injection molding machines, project-specific assembly systems like automated joining systems, gluing apparatus or systems for ultrasound welding) on which the quick and uncomplicated production of small series, development samples, or clinical samples of your point-of-care tests is possible at any phase of the project. The TCCs possesses a clean room in accordance with ISO 14644-1 ISO-classes 7, 8 and GMP-classes C and D, in which the entire value creation chain can be reproduced.
Product-specific production steps for PCR-tests
In addition to the development, the injection molding and the assembly of point-of-care tests made of plastics, we offer our customers product-specific manufacturing steps like, for example, the filling of the dilution solution required for the test or the loading of the plastic housing with the test strips under climate, temperature, and humidity conditions we design for precisely this purpose. Even logistics is tailored to product-specific requirements with the subsequent transport in special vehicles. Due to the extremely high sensitivity of point-of-care tests with regard to contaminants, we offer our customers the entire production and assembly process in the clean room in accordance with ISO 14644-1 ISO class 8, including sealing in an aluminum pouch offering maximum protection prior to shipping.
Worldwide production capabilities for point-of-care tests

As a global player, it is self-explanatory for us to think and act internationally. Our customers therefore have worldwide production facilities at their disposal in Pfreimd (Germany), Horšovský Týn (Czech Republic), Skopje (North Macedonia), Peachtree City (USA), Indaiatuba (Brazil) and Dongguan City (China). With a production area of 120,000 sqm (1,300,000 sqft), of this around 60,000 sqm (670,000 sqft) of clean room area according to ISO 14644-1 ISO classes 7, 8, 9 and GMP classes C and D, as well as more than 350 injection molding machines, we are one of the leading companies in the industry.