Clinical Trial Kit
To accelerate drug development
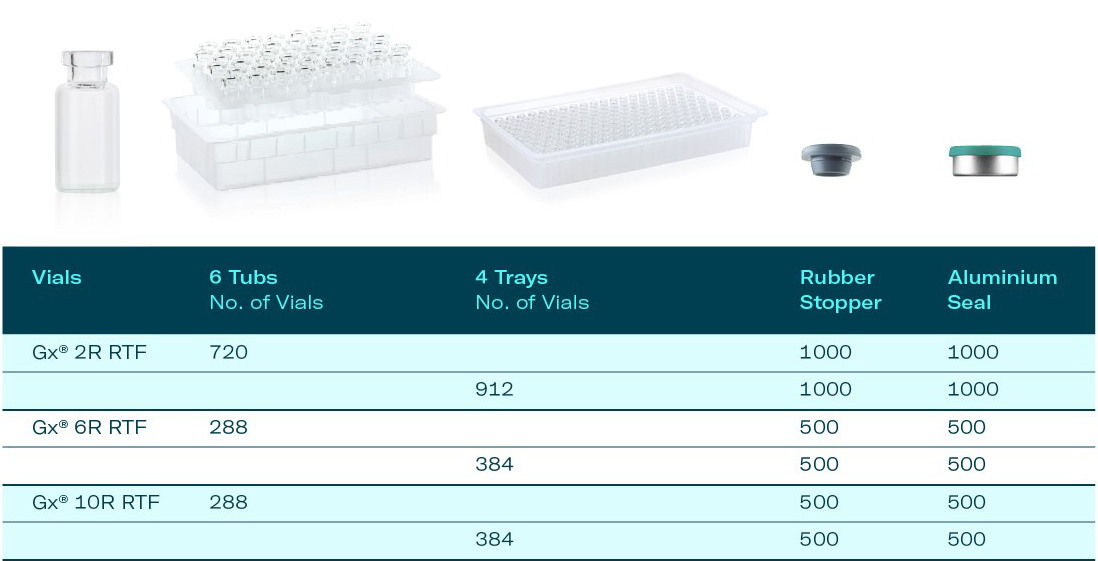
Our Clinical Trial Kit consists of sterile Gx® RTF vials in nest & tub or tray with matching closures and is tailored to support the development of new drugs, vaccines and biologics in early phases. The Gerresheimer Clinical Trial Kit is suitable for small batch manufacturing from first line trials to validation and clinical batches. It can be ordered in six different configurations of Gx® RTF Glass Vials. Kits including Gx® Elite and Gx® RTF COP vials will follow soon.
Benefits and properties
- Sterile Gx® RTF glass vials in EZ-fill nest & tub or tray
- Superior Gx® Elite vials also available in ready-to-fill
- Selected range of stoppers and seals
- All-in-one solutions ready-to-use for small and clinical batches
- Same Gx® RTF vial platform for large scale manufacturing
- Compatible with standard and robotized filling lines
- Reduced time-to-market

Standard configurations and customized kits
The Clinical Trial Kit provides a complete set of primary packaging containers and containment solutions, especially selected for high value drugs and/or most complex and demanding drugs. It complies with GMP requirements to produce clinical batches. Proven product features are ensured with each kit, such as the integrity of the container system.
Currently we offer 6 standard configurations with sterile Gx® RTF vials in nest & tub or tray for filling volumes 2R, 6R and 10R. With our experts’ help, your individual kit can be created from the selected range of rubber stopper and aluminum seals. Kits without stoppers and seals can also be provided.
Additional integrated services
We accompany you from the early phase through to life cycle management. With a network of selected partners we provide expertise regarding regulatory implementation, development path and market approach, strategies for packaging and administration of drugs as well as laboratory services.
Analytical lab services:
- E&L studies (components and systems)
- Material characterization according to ISO 10993-18:2020
- Biocompatibility studies according to 10993 series
- Toxicological risk assessment and consultancy services
- BEP/BER writing services
Formulation, fill & finish
Together with selected partners, we support your journey from clinical batches to ramping-up for commercial supply. No matter if CROs, CMOs or CDMO support is needed, we know the right partner for a wide range of drug products such as vaccines, mAbs, mRNA-based drugs or ATMPs.