Mold making
Precision molds for clean room production of drug delivery systems
For more than 60 years we have been designing and producing sophisticated high-performance injection molds for use in the clean room, whether for the Gerresheimer Medical Systems business unit or for external clients. Our precision molds are distinguished by 100 % repeat accuracy, durability and optimized temperature feed for short cycle times. The experience gained from more than 8,000 constructed molds makes us the ideal partner for precision tools for clean room applications.
Your PLUS with Gerresheimer
High-performance injection molds for use in the clean room
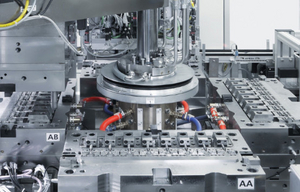
Our high-cavity series molds are designed for the high output quantities and sophisticated quality requirements of the pharmaceutical and healthcare industries. The molds are produced clean room-compatibly for stainless steel. Needle sealing nozzles exclude the formation of threads and thus of particle formation during demolding. The good ventilation of the molded part in the cavities prevents sedimentations that can also come loose in the form of particles. A glide-promoting coating of all movable parts in the mold ensures that no lubricant needs to be used. Adequate demolding angles ensure clean part demolding and avoid abrasion.
Most modern mold technologies
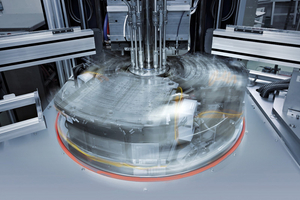
More than 65 specially trained employees produce single-cavity and multi-cavity injection molds with up to 128 cavities, tools for insert molding, indexing plate molds, stack molds, micro-precision molds, rotary table molds and hot-runner injection molds. Our molds are structured modularly and standardized. Exchangeable mold inserts ensure short waiting and repair times without additional adjustment work.
Uncompromising quality assurance
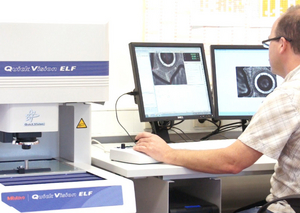
The manufacture of our molds involves the use of the most modern vertical and wire eroding systems, precision grinding machines for all processes and micro-HSC milling machines. Uncompromising quality assurance is the highest priority in the entire production process. Precision molds are ultimately the prerequisite for excellent product quality. This is why only the most modern measuring equipment, for example, CNC image processing measuring machines, are used in the internal measurement lab of Gerresheimer Werkzeugbau Wackersdorf GmbH.
Qualification of molds according to defined SOP's in accordance with GMP and customer requirements
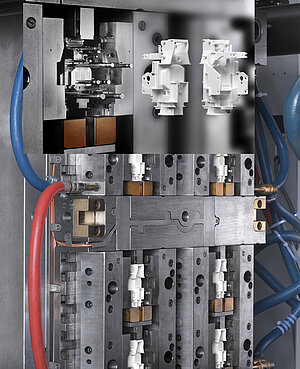
According to the rules of Good Manufacturing Practice (GMP), qualification and validation of the injection molding tools are necessary in every phase of the development and industrialization process.
This starts with the Design Qualification (is the mold design capable of manufacturing the specified products?), through Installation Qualification (does the realized mold correspond with the design?), Operational Qualification (do the products manufactured with the mold comply with specifications?) and finally Performance Qualification, during which the mold is commissioned under production conditions in order to prove that stable series operation is possible with the mold. The qualification service of mold makers often ends with Operational Qualification.
The functional capability of the mold is thereby checked on an injection mold machine of the mold maker, which is in most cases not identical with the machine used later for production. The ambient conditions are also different than those of series production.
Thus, for example, testing doesn't take place under the clean room conditions subsequently prevailing. The consequences are requalifications of the mold that cost time and money after transfer to the production location.
Gerresheimer, on the other hand, offers an integrated qualification and validation process that covers all phases of Design Qualification extending to Performance Qualification. Operational Qualification and Performance Qualification thereby take place under real conditions on the corresponding injection mold machine and in the corresponding production environment.
Technical Center

The technical center of the TCC is our practice-oriented competence center for matters of injection molding. Molds and special-purpose machinery are subjected here to comprehensive application and processing tests under close-to-series production conditions and brought to series-production readiness. The sampling and mold optimization process in the technical center forms the basis of the entire component verification. Important stages of this process include the creation of a stable configuration setting for injection molding and comprehensive component measurement documented in an extensive initial sample test report. Machine and process-capability documentation and mold trials over defined periods of time (e.g. 4 or 24-hour runs) complete the technical center phase.

