Düsseldorf/Venice (Italy). March 13, 2019. With its Gx Elite and Gx RTF vials, Gerresheimer is presenting two new type I vials made from borosilicate glass at booth 115 at the PDA Annual Meeting March 19-20) at the Hilton Stucky Molino, Venice, Italy. Robert Hayes, Senior Director Innovation & Product Management, will speak about the industry’s top challenges related to the Quality of Specialty Pharmaceutical glass packaging and share recent innovation developed by Gerresheimer, introducing Elite Glass Products.
“These high-end tubular glass vials made of type I borosilicate glass are our response to increasingly stringent customer demands and expectations on the pharmaceutical market and greater demands for patient safety,” says Robert Hayes, Senior Director Innovation & Product Management, in his presentation, emphasizing the extent to which manufacturing improvements and avoiding glass-to-glass contact in the production process can affect the quality of the vials.
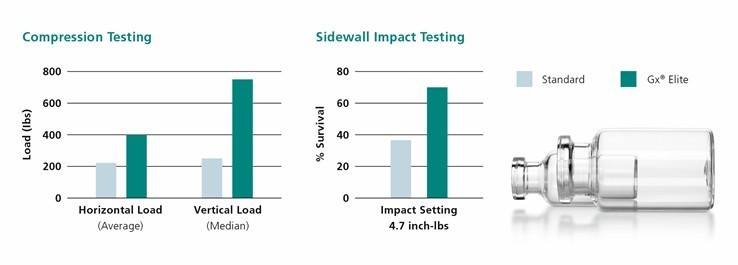
Extremely durable and free of cosmetic defects
The Gx Elite vials are the result of a careful product development pro-cess spanning several years. The primary focus for Elite Glass products was to provide a safer product for the customer and patient. This effort has impressed our customers,” The highly shatter-resistant vials are extremely durable, free of cosmetic defects and can be customized for specialized customer requirements. Elite Glass vials are produced using the quality by design approach that creates a product that will exceed customers’ expectations. A combination of manufacturing and handling improvements along with specialized final packaging also ensures that Gx Elite vials can be supplied for end-to-end use on various filling lines. These types of improvements make it possible for our customers to sup-ply products to the market as needed and reduce costs for the industry, which will ultimately help everyone.