Full transparency right from production to point of care
The Gerresheimer traceability concept ensures complete transparency throughout the value chain and greater safety for the patient — because only complete traceability is true traceability. As the first link in the manufacturing chain, we apply unique codes to our primary packaging and medical devices. Thanks to our digital infrastructure for traceability, we and all actors in the pharmaceutical supply chain can link, analyze and share any data along with the individual product unit. Our codes are the key to connecting previously separate worlds in the value chain. They mark the start of a new era of interconnectedness that promises complete transparency and enhanced security for our customers and patients.
Benefits of traceability
Our vision promises transparency and traceability across the entire supply chain, from primary packaging to secondary packaging, and from drug filling and serialization up to the final application at the PoC. Traceability helps to:
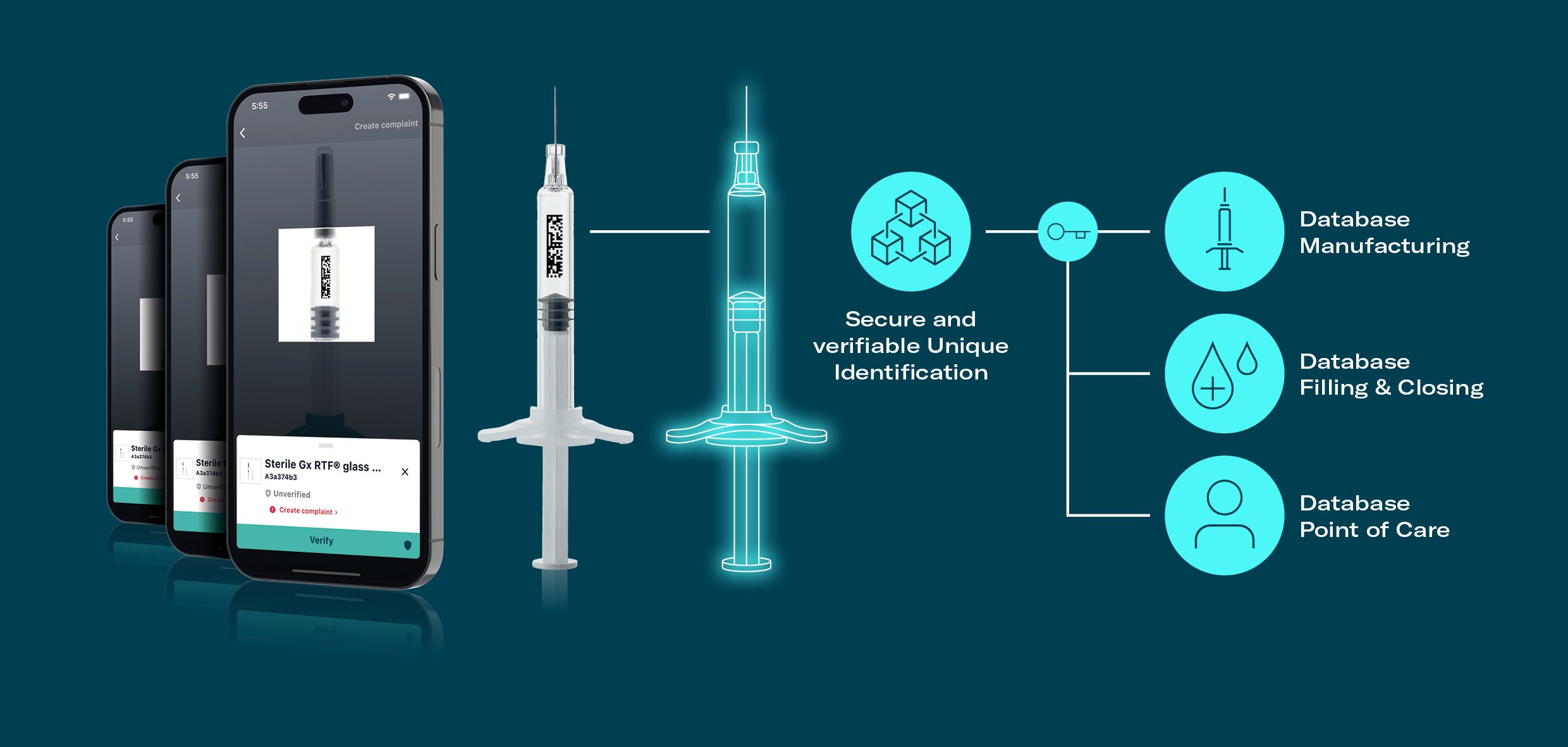
Digital Twin Solution
Gerresheimer is working to revolutionize its primary packaging by transforming it into a secure gateway to access digital twins.

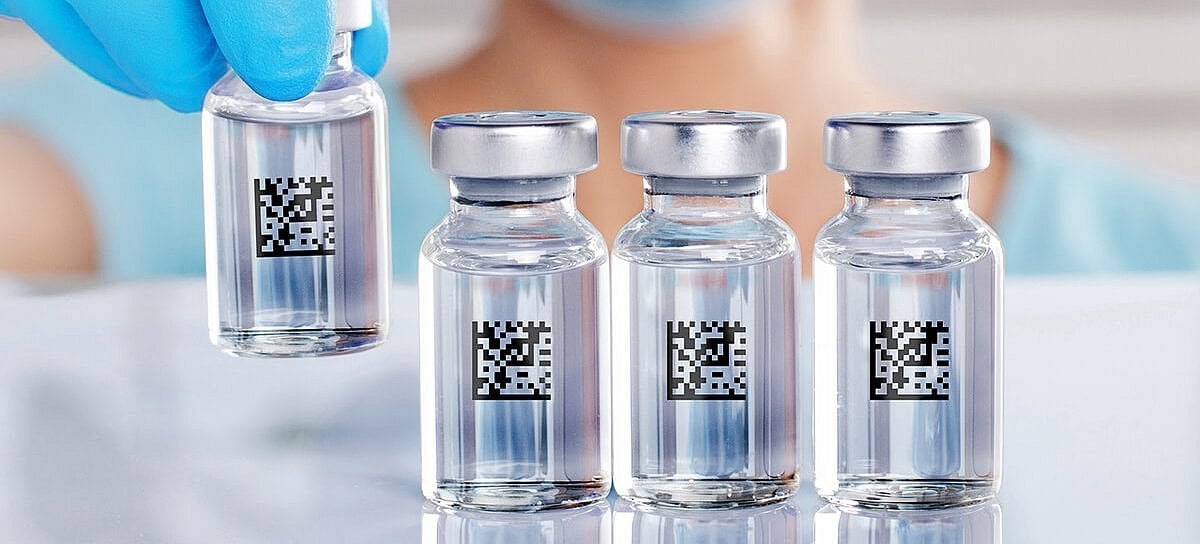
Container-level Identification
Gerresheimer offers a choice of marking technologies for its broad product portfolio.