Düsseldorf/Barcelona, October 4, 2016: On the occasion of this year's CPhI, the company presented the first product of its newly developed Gx RTF ClearJect brand: a COP syringe with cannula. The prefillable, high-performance plastic COP (Cyclo-Olefin-Polymer) syringes made in Europe are especially suitable for demanding, sensitive medications and high-viscosity agents. In the future, American and European customers will be offered a portfolio of COP syringes from a single production location in Europe. The new development reflects the combined know-how of experts in prefillable glass syringes in Bünde, Germany and the plastic specialists at the Technical Competence Center in Wackersdorf, Germany.
Gerresheimer currently offers a range of prefillable COP syringes produced by long-time company partner Taisei Medical Co. Ltd. in Japan. Gerresheimer is assuming the sales and technical consulting roles for ClearJect syringes for customers in Europe and the US. The company is now expanding its product portfolio of COP syringes and is combining the tried-and-tested RTF (ready-to-fill) concept of glass syringes with ClearJect to create the new Gx RTF ClearJect brand. In close cooperation with the company's Japanese partner, the new syringe will be produced at the German production facility of Gerresheimer Medical Systems. The first product of this line is a 1 ml long syringe with integrated cannula.
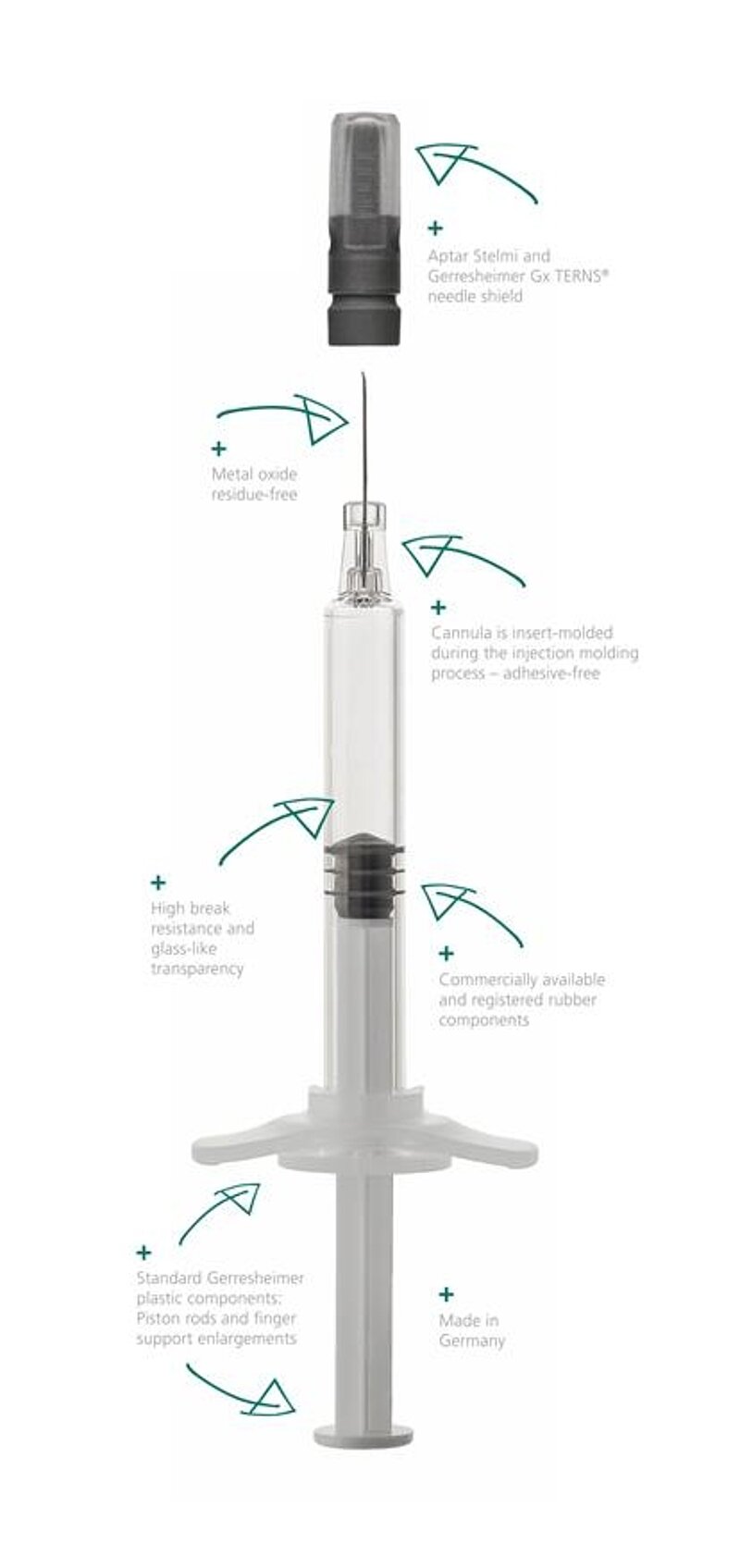
COP is an interesting plastic alternative to time-tested glass syringes due to the growing demands of novel agents on their primary packaging. Medications for cancer therapy, for example, can be extremely aggressive to the point where the break resistance of a syringe is a decisive criterion for selection. Innovative biotech medications, on the other hand, are often effective in the smallest of doses and are frequently very expensive. Any interaction with the syringe material must be ruled out here. COP meets all of these requirements. Syringes made of this material are resistant to breaking, as transparent as glass and hardly interact with the packaged medications at all. Thanks to the use of injection molding, the design boasts especially tight tolerances. Its precise geometry also reduces dead volume, leaving behind less of the expensive medication in the syringe.
The new Gx RTF ClearJect syringe with cannula offers key advantages with regard to the primary packaging of demanding medications, especially when it comes to biocompatibility. The COP material does not release metal ions into the medication solution. Since the entire syringe including the insert-molded cannula is produced in a single step, the product is also free of tungsten and adhesives. COP has a high pH tolerance and, unlike glass, does not change the pH value while in storage. The oxygen permeation rate is low in comparison to other plastics, and the values for extractables and leachables are low. The syringes are siliconized with a precisely controlled amount of high-viscosity, and thus low-particle, Dow Corning 360 MD (12,500 cSt) silicone oil to ensure optimum functioning.
Another important advantage of the Gx RTF ClearJect syringe with cannula is its end-user safety. COP is particularly break-resistant, making it suitable for packaging aggressive or toxic materials.Gerresheimer chose COP as the material for its new syringe due to these superior physical properties. Precise dimensions and siliconizing ensure reliable syringe functioning with low breakaway and gliding forces and minimal force required to pull-off the needle shield. The syringes are also excellently suitable for use in autoinjectors thanks to their ruggedness and precise geometry.
This syringe system is economical thanks to the fact that the  innovative COP syringe body is designed to use commercially available components throughout. This begins with the use of standard cannulas and continues with the piston rods, piston plungers, backstops and closure systems which can be used.
innovative COP syringe body is designed to use commercially available components throughout. This begins with the use of standard cannulas and continues with the piston rods, piston plungers, backstops and closure systems which can be used.
The new Gx RTF ClearJect syringe is available in the 1 ml long size. The design is inspired by ISO 11040-6 and registered. The syringe is equipped with a 27-gauge, 1/2-inch (12.7 mm), thin-walled stainless-steel cannula with three bevels.
About Gerresheimer
Gerresheimer is a leading global partner to the pharma and healthcare industries. The company’s special glass and plastic products contribute to health and well-being. Gerresheimer is a global organization with 11,000 employees and manufacturing operations in the local markets, close to customers. It has over 40 production facilities in Europe, North and South America and Asia generating revenue in excess of EUR 1.4 billion. The comprehensive product portfolio includes pharmaceutical packaging products as well as convenient and safe drug delivery systems such as insulin pens, inhalers, pre-fillable syringes, vials, am poules, bottles and containers for liquid and solid pharmaceuticals with closure and safety systems, plus cosmetic packaging products.
Gx is a registered brand of the Gerresheimer Group.
RTF is a registered brand of Gerresheimer Bünde GmbH.
ClearJect is a registered brand of Taisei Medical Co. Ltd.