Düsseldorf/Paris January 08, 2020. Which glass and plastic primary packaging and which administration systems are suitable for newly developed drugs, such as biologicals, and therapies? What do chronically ill patients need to be able to take care of themselves reliably on an ongoing basis? These and many related questions will be answered by Amir Tahric and Dr. Wenzel Novak in their workshop at Pharmapack on February 6 at 10 a.m.
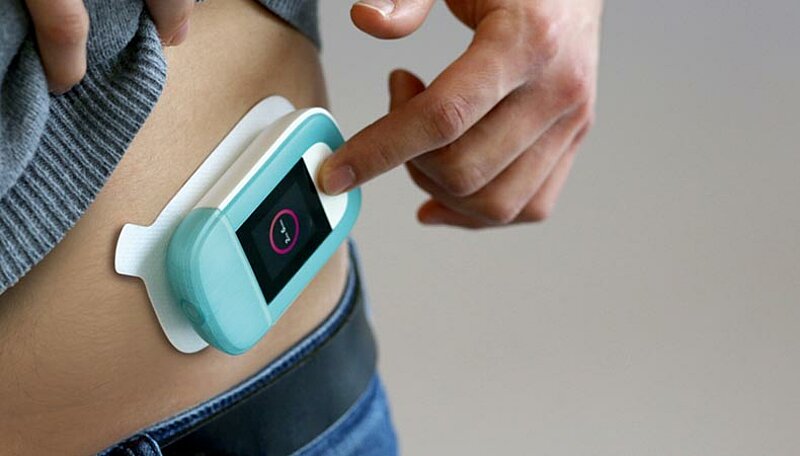
The pharmaceutical industry is focusing on new therapies. Personalized treatment such as cell therapies, new biomolecules, and drugs for rare diseases are playing a key role in new approaches. This is why smaller batch sizes are often requested for filling. In addition, bottled products are sensitive to their storage environment, the container. Containers therefore need to be individually adapted to the application. The ongoing shift of treatment away from hospital and toward the patient’s home relies on user-friendly administration systems like injection pumps.