Customized Drug Delivery Systems for Inhalation Therapy
In close cooperation with leading pharmaceuticals companies, Gerresheimer Medical Systems has been developing and producing powder inhalers, capsule inhalers and nebulizers for the treatment of respiratory illnesses like asthma, COPD (Chronic Obstructive Pulmonary Disease) and cystic fibrosis for more than 20 years.
Your PLUS with Gerresheimer
Experience with more than 100 million inhalers produced annually

With our annual production of more than 100 million inhalers, we are a leading specialist in the field of customer-specific inhalers for inhalation therapy.
Full Service: from concept development to the series production-ready product
As a full service provider, we assume responsibility for all stages of the value creation chain, from planning to ready-for-sale products: Concept development, industrial design, product development, production equipment design, mold making and special-purpose machinery engineering to large and small series production in the clean room according to ISO 14644-1 ISO classes 7, 8 and 9 under FDA/GMP conditions, the assembly of modules, as well as the pharmaceutical assembly and filling, sterilization and packaging – at Gerresheimer Medical Systems your receive all services from one source.
Design for Manufacturing: short time-to-market

Gerresheimer Medical Systems develops its products according to specifications or optimizes the component layout for injection molding production on the basis of your ideas. Our engineers thereby already consider the design for manufacturing in the development phase of the inhaler. For you this means a reduction in the development time, the development costs and the project risk. Our experts for design and development work hand in hand with special-purpose machinery engineering. In this way we ensure that the individual injection molding parts and assembly units can be assembled without problems following production and that optimal functional safety is guaranteed.
Test samples for clinical test studies and development samples

We offer you another clear gain of time in the development phase with our small series production in the clean room in accordance with ISO 14644-1 ISO-class 8. Here we produce small series as test samples for clinical test studies, upon request including filling. Our range of services also includes the assessment of inhalers and the evaluation of systems, including the complete documentation (e.g. Device Master File) as the basis for the approval of the product.
Continuous cleanliness chain

The entire value creation chain, from the precision injection molding in large and small series production through fully automatic, semi-automatic and manual assembly of plastic parts, assembly units and metal parts extending to the product refinement and packaging takes place in the clean room according to ISO 14644-1 ISO classes 7 and 8.
Highest quality through 100% in-process inspection

Our internal special-purpose machinery engineering conceives of and builds assembly systems for a highly precise feed of plastic and metal parts at the highest speed. At the same time, we ensure the optimal surface quality and functional safety of the products through assembly that is gentle with the visible surfaces and visual inspection for damage to the visible parts within the assembly system. A 100% in-process inspection of the assembly steps, of the assembly units and product functions takes place with a large number of intelligent camera systems and inspection stations in the assembly system.
Installation of the electronics in inhalers
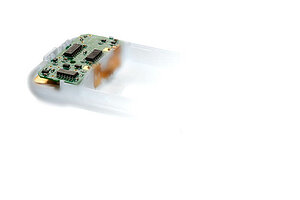
As an expert for the combination of plastics and metal, we also assume responsibility, upon request, for the assembly of the electronics in inhalers: electronic counters for dosing, atomization actuators and the most varied sensors. We integrate punch contacts and install circuit boards, which are then, for example, designed as switches. For example, a volume switch detects the correct inhalation of the patient during inhalation and triggers the application of the medication. We create our own clean rooms for the assembly of electronics. These are also equipped with a special ESD protective floor for preventing static charge. Special camera tests in the assembly system ensure the correctly positioned insertion of circuit boards, the damage-free positioning and snap-fitting of punch parts and the correct position of switches. A gap-free in-line function test at the assembly system rounds off our range of services.
Modern injection molding technologies: micro injection molding

Miniaturization is one of the most important technological trends. An increasing number of functions are being integrated into inhalers, resulting in the components needing to become ever smaller and requirements for precision increasing. In order to fulfill these requirements, we use the most modern injection molding technologies for the production of inhalers, like micro injection molding.
Successful FDA audit of inhaler production in China

Our location in China, Gerresheimer Medical Plastic Systems Dongguan Co., is an FDA-inspected medical device manufacturer for the production of an inhaler for the treatment of cystic fibrosis. On the context of the drug approval of an inhaler for the American market, the entire value creation chain was examined closely in accordance with strict standards. This includes the purchasing of rubber and metal parts, the injection molding of the plastic parts for the inhaler, the assembly of the inhaler plastic parts and testing, the laser printing and the seal of originality. Injection molding, assembly, testing and sealing of originality take place in the clean room according to ISO 14644-1 ISO class 8.
Worldwide clean room production

We produce inhalers worldwide – in Pfreimd (Germany), Horšovský Týn (Czech Republic), Peachtree City (USA) and Dongguan City (China). The production of inhalers presumes adequate ambient conditions. This is why Gerresheimer Medical Systems offers around 60,000 sqm (670,000 sqft) of clean room area worldwide in accordance with ISO 14644-1 ISO classes 7, 8, and 9 and GMP classes C and D.